Introduction
REQUEST FOR APPLICATION
NewCures Accelerator: Drug Target Advancement Program (up to $50k)
November 2021
NewCures is Northwestern University’s biomedical accelerator that focuses on advancing the development of novel therapeutics. NewCures supports projects through pre-clinical stages, in particular, proof-of-concept validation and pharmacological de-risking. By aligning with industry standards, NewCures fills a translational gap in therapeutic development and aims to enhance and de-risk discoveries by:
- identifying potential assets in faculty laboratories;
- providing expertise and resources to formulate a strategic, project-specific development plan;
- developing robust, validated preclinical data; and
- translating research to industry-ready assets to attract external partnerships and investment from the private sector.
For the 2021 Request for Application (RFA), the focus is Target Advancement, such as Target validation, Target Optimization and Target Prioritization, to translate basic research and enable novel therapeutic development. To this end, NewCures is seeking early-stage studies that can bridge the critical gap between basic biology and drug development. The proposed studies will build robust evidence to rationalize biological pathways and targets for further translation into new therapeutics.
For this round, NewCures will not consider proposals focused on validation of established targets or proposing discovery studies to identify new targets (i.e., large-scale screening or genomic/transcriptomic analyses).
Contact Information
NewCures@northwestern.edu
Key Dates
Applications due: November 22, 2021
Area of Interest
All molecular-based therapeutic projects with an identified biological target with a translational/commercialization trajectory are encouraged to apply. At this time, NewCures does not support projects involving therapeutic delivery/formulation, diagnostics, devices, research tools or healthcare information.
Eligibility
Applicant: Northwestern University principal investigator (PI) with a full-time faculty appointment.
Intellectual property (IP) related to the application must meet the following criteria (if applicable):
- Owned by Northwestern University;
- If IP is jointly owned, NU must be the lead, and joint owner must be a non-profit organization;
- Not licensed to a third party.
If IP has not been disclosed to INVO, please contact INVO and NewCures to discuss. All projects will be reviewed for IP protection for NewCures eligibility. The invention disclosure process is on INVO website (http://www.invo.northwestern.edu).
Funding/Benefits
Projects will be supported at a level deemed necessary to achieve carefully evaluated objectives. After project selection and PI agreement, NewCures will:
- Work with the PI and external experts to formulate a development plan, outlining key experiments for proof-of-concept validation;
- Execute milestone-driven project plans, which will mainly consist of outsourcing experimental work to third party contract research organizations (CROs) – outsourced work will be managed and paid for by NewCures (up to $50K)*; and
- Work with INVO and the PI to build IP portfolio (if applicable) and engage potential private sector partnership and/or investment, contingent on the achievement of desired experimental outcomes/milestones.
*NewCures serves the role of project manager and makes direct payments to the CROs. NewCures does not provide direct funding to a PI’s laboratory (NU chartstring).
Evaluation Criteria
The goal of NewCures Accelerator is to advance molecular-based technologies to the point where technology transfer is achieved or additional funding/partnership is secured from the private sector. Thus, applications will be evaluated on overall potential for technology transfer, including scientific/technical merit, unmet medical need and commercial potential.
Please refer to Table 1 (may not apply for ‘Drug Target Advancement Program’) for a list of evaluation criteria for project review. The gaps identified will be noted as potential areas of support by NewCures once the project is selected for NewCures development processes. Importantly, NewCures does not expect that submitted projects will have the data/information to support all criteria listed below.
Table 1. Example list of criteria for NewCures Review and Development
|
Target Validation
|
Evidence of target engagement
Assessment of off-target activity
|
|
Scientific Rationale
|
Mechanism of action
Biological Data/Efficacy
– In Vitro
– In Vivo animal data
Safety demonstration
|
|
Novelty of Composition
|
Screening of Hit-to-Lead
Identification of lead compound
|
|
Drug-like Properties
|
Pharmacokinetics/Pharmacodynamics
Protein binding
Metabolic stability
|
|
Attractiveness of Indication
|
Potential impact and significance for patients
Market need and opportunity
|
Application Directions
Send the application as a single PDF file to newcures@northwestern.edu by 5:00 PM CST on November 22, 2021. Please do not include appendices.
Prepare a project description (1 title page, 2 project pages including figures and references, 11-point font, 1” margins) as follows:
Title Page (1 page)
- PI name and affiliation
- Project title
- Project team (if applicable): name, affiliation and area of expertise of the research scientists
- Project area: small molecules, biologics, gene therapy, protein therapy, cell therapy, others
- Intellectual property: list if there are patents covering the project or invention disclosures with INVO, i.e., Northwestern reference number
- All prior, current and pending sources of support related to the application
- IRB/IACUC approval number (if applicable)
- Non-confidential executive summary (≤ 150 words), which may be shared with industry partners with permission from the PI: describe the biomedical need or opportunity and the significance of the proposed technology; state the major research findings and how they will advance therapeutic potential; describe the potential commercial market for the technology
Project Pages (2 pages)
- Project description and clinical need
Summary of the project including:
i. Scientific merit and significant findings
ii. Current stage of development
iii. Description of the medical need and desired indication
- Competitive landscape
Brief description of the current standard of care and how the potential therapeutic of this project, if developed, will be an improvement over currently available treatment
- Project needs
Briefly summarize the resources and expertise that are needed to progress the project towards drug development with an estimated cost. Please note NewCures does not provide funding towards personnel, equipment, materials or reagents (see Funding/Benefits tab).
APPLICATION DEADLINE: MONDAY, NOVEMBER 22, 2021, 5:00 PM CST
NewCures@northwestern.edu
|
Review Process
Each application will be judged on the basis of translational and commercialization potential, scientific merit, and medical need.
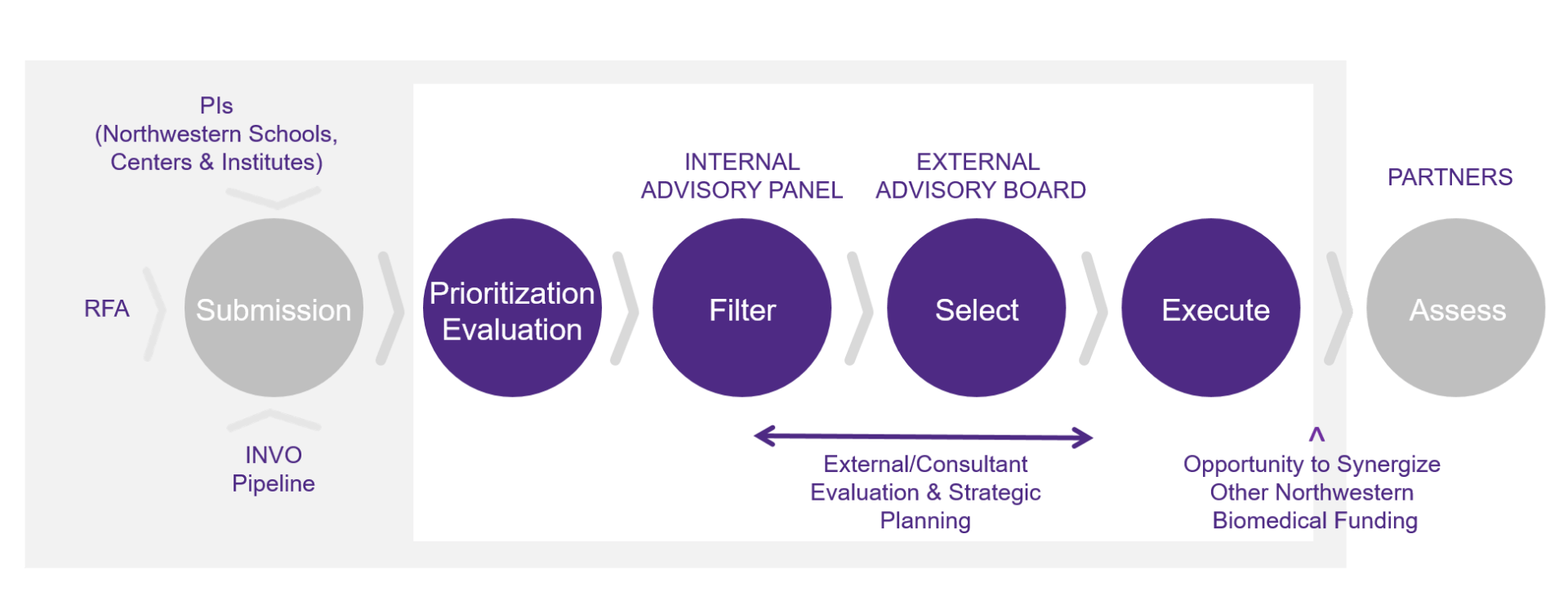
Applications will be initially reviewed for basic eligibility and feasibility, followed by a review and prioritization by NewCures and INVO team members experienced in therapeutic technology assessment and development. NewCures may request for additional data and information during the prioritization process. Prioritized applications will be presented to the NewCures External Board of Advisors (EAB) for final evaluation.
NewCures EAB consists of experts from outside of the Northwestern community who are highly recognized leaders in therapeutic development from pharmaceutical, biotech and investment communities; all external members have signed confidentiality agreement with Northwestern. Only external members will be involved in the final selection and funding of NewCures projects to avoid a potential conflict of interest.
Project Execution and Management
NewCures project team will work with the PI, external consultants and subject matter experts to develop a pre-clinical development plan for the technology. The experimental work will be coordinated and managed by NewCures, either through internal resources (Northwestern Core Facilities) or third party CROs.
NewCures and INVO will work with the PI to generate a “pitch deck” to facilitate engagement with the private sector for potential licensing, collaboration and/or investment.